Absorption and Adsorption
- No Comments
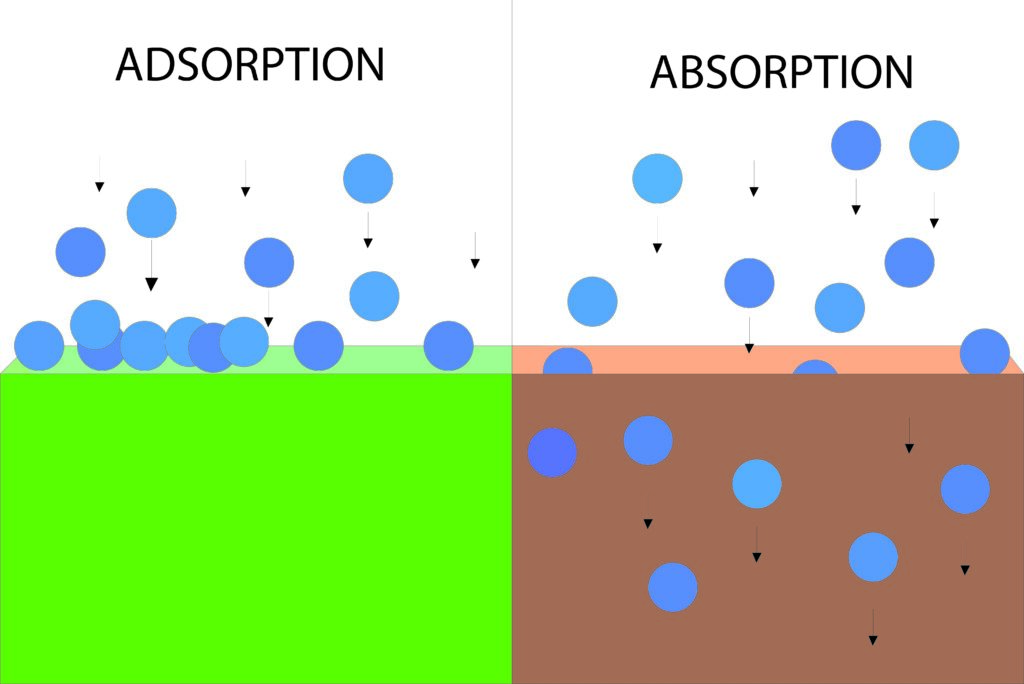
Adsorption and Absorption are two fascinating phenomena that play a crucial role in a variety of fields, from chemistry to environmental engineering, from pharmacy to the food industry. They accompany us daily, and although they may sound similar, they are fundamentally different. In this blog post, we will delve into the world of Adsorption and Absorption, clarify their definitions, explore their mechanisms, and learn some insightful examples of their applications.
Definitions
- Absorption:
Absorption is the process in which one substance penetrates and dissolves in another substance. This typically occurs in liquids or solids when a gas or liquid is taken up by the surrounding phase. - Adsorption:
Adsorption is a broad term that describes the process in which molecules or ions from a gas or liquid phase are bound to the surface of a solid material.
What is Absorption?
Essentially, absorption means that a substance is taken up by another, with the absorbed substance being evenly distributed in the absorbing phase. Typically, this process occurs in liquids or solids and results in the formation of a homogeneous solution or dispersion. This phenomenon can occur in various environments, including gas absorption in liquids or liquid absorption in solids.
Mechanism and Examples of Absorption
The mechanism of absorption depends on the substances involved and the conditions. In many cases, absorption is described by Henry’s Law, which relates the concentration of a dissolved gas to the pressure of that gas in the gas phase. As the pressure of the gas above the liquid increases, the amount of gas dissolved in the liquid also increases.
Examples:
- Environmental Engineering:
In environmental engineering, absorption is used to remove harmful gases such as sulfur dioxide (SO2) and carbon dioxide (CO2) from industrial emissions. Absorption solutions like sodium hydroxide or ammonia are often used. - Food Industry:
The food industry uses absorption to bind flavors and odors in food. For example, coffee can absorb flavor compounds from the air, influencing its taste. - Pharmacy:
In pharmacy, absorption is used to formulate drugs into a suitable form for the body. Medications can be absorbed into tablets or capsules to enable controlled release in the body.
What is Adsorption?
Adsorption is a physical or chemical process in which atoms, molecules, or ions from a gas or liquid phase adhere to the surface of a solid material. Essentially, adsorption means that molecules or ions stick to the surface of a material without penetrating the material itself.
Mechanism and Examples of Adsorption
The mechanism of adsorption involves interactions between the adsorbed molecules or ions and the surface of the solid material. These interactions can be of a physical or chemical nature. In physical interactions, also known as weak adsorption, adsorption occurs due to Van der Waals forces. These forces are weak attractive forces between molecules due to temporary charge differences. In chemical interactions, also referred to as chemisorption, genuine chemical bonds form between the adsorbed molecules and the surface of the material. Among many models used to describe adsorption, the Langmuir isotherm is scientifically recognized and utilized for monolayer adsorption (often chemisorption), while the BET (Brunauer-Emmet-Teller) model is employed for multilayer adsorptions (physisorption).
Examples:
- Environmental Engineering:
Activated carbon is used in industrial air purification systems to remove harmful gases and odors. Activated carbon adsorbs gases like carbon monoxide (CO), sulfur dioxide (SO2), and volatile organic compounds (VOCs), leading to improved air quality. - Food Industry:
Silica gel, a porous adsorbent material, is used in food packaging to adsorb moisture, thereby preserving the shelf life and quality of foods like dried fruits. Silica gel can also adsorb flavors, preserving the taste of foods. - Pharmacy:
In pharmaceutical production, adsorption is used to remove unwanted impurities from drugs. Ion exchange resins, for instance, can be used to adsorb contaminants like heavy metals from a drug solution, ensuring the drug’s purity.
Everyday Examples of Absorption and Adsorption
As mentioned earlier, absorption and adsorption are present in our everyday lives. Here are some everyday examples:
Absorption:
- Dishwasher Detergent Tabs:
The tabs we use in dishwashers often contain absorbing components. The surfactants in the tabs can absorb grease and dirt particles as they dissolve in water, enhancing cleaning efficiency. - Sponges and Cloths:
When we use a wet sponge or cloth, they absorb water by storing it in their pores, allowing us to clean surfaces and remove dirt. - Coffee Filters:
Coffee filters are made of porous paper that absorbs water while retaining coffee oils and particles. This clarifies the coffee and enhances its aromatic flavor.
Adsorption:
- Odor Removers:
Many household odor-eliminating products, such as activated carbon filters or air-based odor removers, utilize adsorption. These materials adsorb unpleasant odors and impurities from the air, purifying the air. - Kitchen Dishcloths:
Kitchen dishcloths and cleaning cloths can use adsorption to remove grease residues and food particles from surfaces. The adsorptive properties help keep surfaces clean. - Dehumidifiers:
Dehumidifiers in our homes use adsorption to remove moisture from the air. The contained adsorbent material attracts and traps water vapor, reducing room humidity. This helps keep the air dry and prevents mold and condensation.
Conclusion
Absorption and Adsorption are versatile processes that play a central role in environmental engineering, the food industry, and pharmacy. They already have a strong impact on our daily lives, whether in air purifiers, food packaging, or medications.
The future holds even more potential. Through continuous research and the development of innovative absorbing and adsorbing materials, we may find solutions to pressing environmental issues. Moreover, new applications could emerge in fields such as renewable energy and medicine.
Absorption and Adsorption are key processes in shaping a more sustainable and efficient future.
Sources:


